An Integrated Genomics and Clinical Resource for Data-Driven Health Services Policy and Practice Decisionmaking
1. Cancer Control Research Program, BC Cancer Agency, Vancouver, British Columbia, Canada; 2. Molecular Oncology, BC Cancer Agency, Vancouver, British Columbia, Canada; 3. Radiation Oncology Program, BC Cancer Agency, Vancouver, British Columbia, Canada
Background: Biomedical understanding and approaches to cancer are evolving rapidly. Scientists are decoding the genomes of different cancers and linking these to an array of biological and medical/clinical data about the progression of the disease in different patients. Stratified treatments based on genetic information have been developed, particularly for breast, colorectal and skin cancers, and there are a wide array of clinical trials in progress to develop and enhance future treatments. Post- genomics cancer research is also being taken up in screening and prevention programs to better identify and manage the risks of cancer. As cancer survival improves, and many survivors face increased risks for long-term morbidity and premature mortality, related directly to the cancer itself, to preexisting comorbidities, or to exposure to therapy, and research is being conducted on genetic predictors of survival, morbidity and quality of life.
In order to translate these findings into effective and efficient healthcare throughout the cancer care trajectory, risk stratification, based on a comprehensive set of genetic, clinical, sociodemographic, and health system factors, is needed, to classify cancer survivors into different levels of intensity and settings for follow-up care to deliver targeted, sustainable care within specific health systems.
Methods and Results: As part of a CIHR-funded grant, at the British Columbia Cancer Agency (BCCA) we have linked population-based, longitudinal, individual registry, clinical, and health administrative records for the approximately 32 thousand breast cancer patients diagnosed to BC residents from 1989-2011, and followed to end 2013, in order to assess long-term (up to 24 years post-diagnosis) mortality, morbidity, healthcare utilization, access and quality of care, and predictors of these outcomes. For a subset of approximately 1000 of these patients diagnosed between 2005 and 2009, CIHR has funded collection of biological samples (either blood or saliva) from which DNA was extracted, and a detailed questionnaire, with information on education; ethnicity; health, medical and reproductive history; family history of cancer; lifestyle characteristics; as well as lifetime occupational and residential histories. Tissue microarrays are being created from the tumour blocks. Genotyping using the 600K marker Oncochip is also being performed. For another subgroup of approximately 720 patients, data is available on ten genetically-distinct molecular subgroups with different survival rates, including a high- risk, estrogen-receptor-positive 11q13/14 cis-acting subgroup and a favourable prognosis subgroup without somatic copy number aberrations. This resource is available for research into multiple long-term patient (survival, second cancer and other morbidity) and healthcare (healthcare utilization, compliance with evidence-based care, sustainability of care) outcomes. A strength of this resource is the detailed clinical and treatment information available for the majority of patients, through the BCCA Breast Cancer Outcomes Unit.
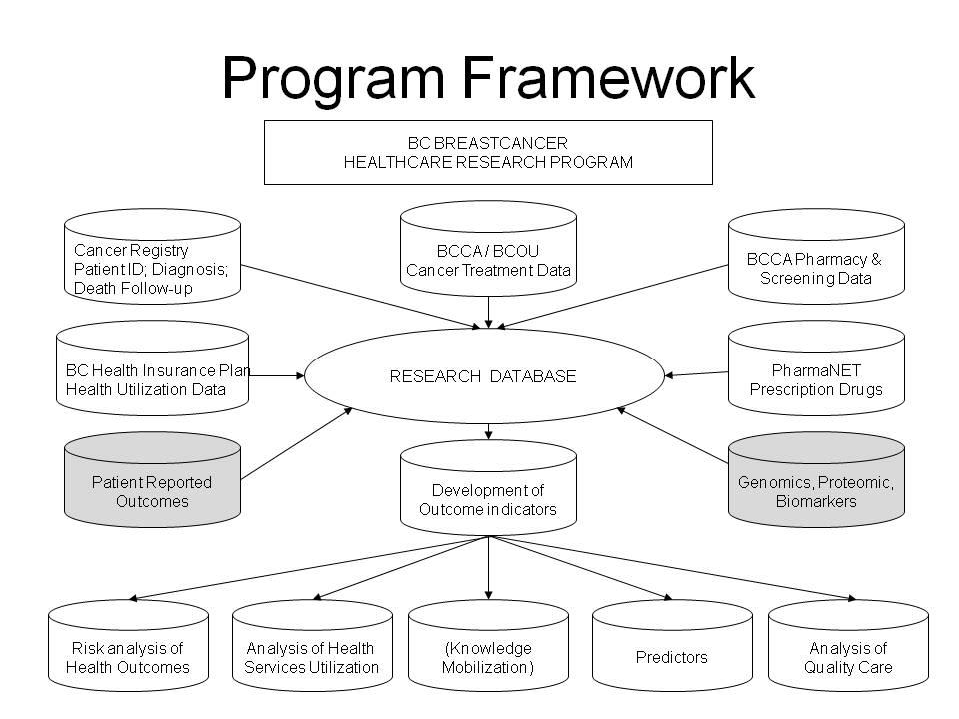
Conclusions: Among oncologists and other cancer specialists, this work will raise awareness of issues; identify treatment toxicities; contribute to clinical decision-making; inform survivor care guideline development; and encourage research into treatment alternatives. Among family physicians and other care providers, this work will raise awareness of risks of late complications among their patients; identify high-risk survivors; and support targeted risk-based care in primary care. Among policymakers and program managers, findings will identify resource issues; and support cost-effective models of care. And, among cancer patients, this work will raise awareness of the implications of genomics on their long-term care.
